Affinity Biosensors of Santa Barbara receives FDA approval of LifeScale AST testing device

SANTA BARBARA, Calif. – Affinity Biosensors of Santa Barbara announced Wednesday that the diagnostic company's LifeScale AST system has received clearance from the U.S. Food and Drug Administration.
The LifeScale AST system is a benchtop diagnostic platform that provides rapid phenotypic antimicrobial susceptibility tests (AST), for the management of bacteremia in the bloodstream.
Bacteria are naturally found in human blood and most won't make you sick, but some bacteria can trigger your body's immune system response and even lead to an infection.
Healthcare providers can prescribe antibiotics to treat these infections, but there is a risk of antibiotic-resistant bacteria, so knowing what to give to patients and when is crucial in keeping people safe.
Below is a depiction of the LifeScale AST in action.

Key features of the newly approved device are listed below:
- Rapid test results which reduce the current time needed for health providers to pick the best antibiotic therapy
- High Accuracy from using a microfluidic sensor to measure thousands of individual bacteria
- Streamlined workflow from a user-friendly interface and automated processes which allows healthcare professionals to focus more on patient care
The CEO of Affinity Biosensors, Dr. Ken Babcock, said "We are very proud of achieving FDA clearance for the LifeScale AST system. This revolutionary technology has the potential to transform how infections associated with sepsis are treated. We are very grateful to our dedicated team and partners who have worked tirelessly to bring LifeScale AST to market. We are especially gratified to witness this performance borne out in our collaborations with healthcare institutions nationwide."
Below is a comparison of testing timelines using the LifeScale AST system and conventional practices.
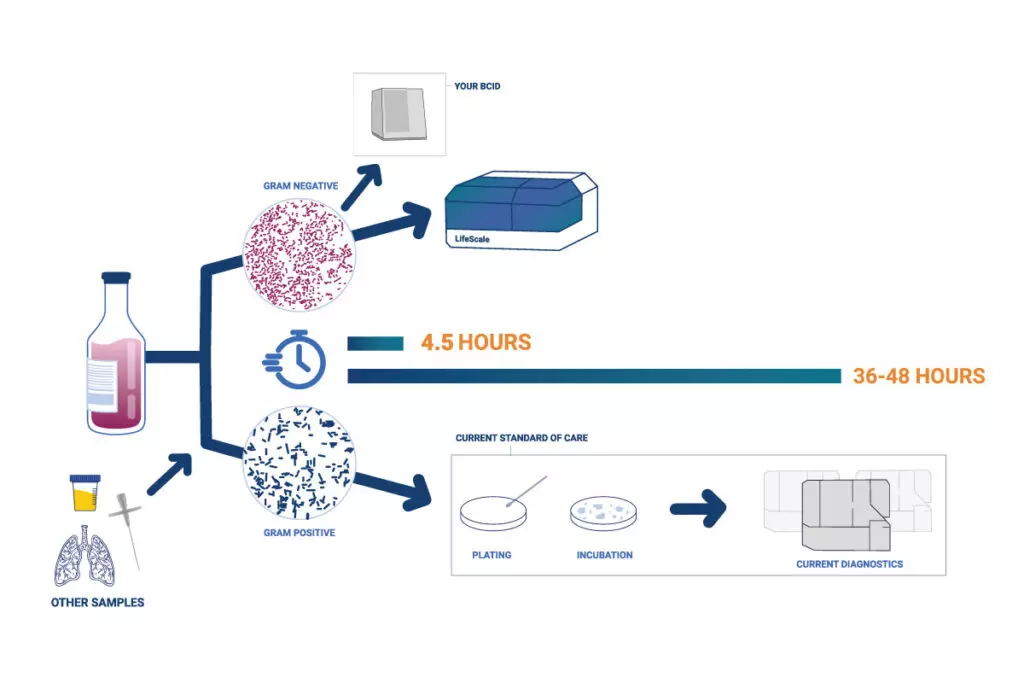
According to a press release from Affinity Biosensors, the device will help healthcare providers make informed decisions, reduce the current duration and cost of treatment, and support proper antibiotic stewardship.
Besides the faster test results, the LifeScale AST features chemistry-free sample preparation, compatibility with existing organism identification systems, and convenient room-temperature consumables storage detail Affinity Biosensors.
Affinity Biosensors was founded in 2006 and since 2014, has focused its mass-sensing technology on multi-drug resistant infections explain the diagnostic tool development company.
